The groundbreaking health feature will roll out as part of the watchOS 26 update
Apple has received clearance from the US Food and Drug Administration (FDA) for its new hypertension notification feature for the Apple Watch.
The news is a significant validation for the company’s health ambitions and arrives just in time, as the feature is set to roll out to users with the launch of watchOS 26 (from today, 15 September).
The groundbreaking feature was a headline announcement at Apple’s hardware event last week, presented as one of the key additions of the all-new Series 11 and Ultra 3 (which launch on 19 September). It will also roll out to existing users of the Series 9, Series 10, and Ultra 2.
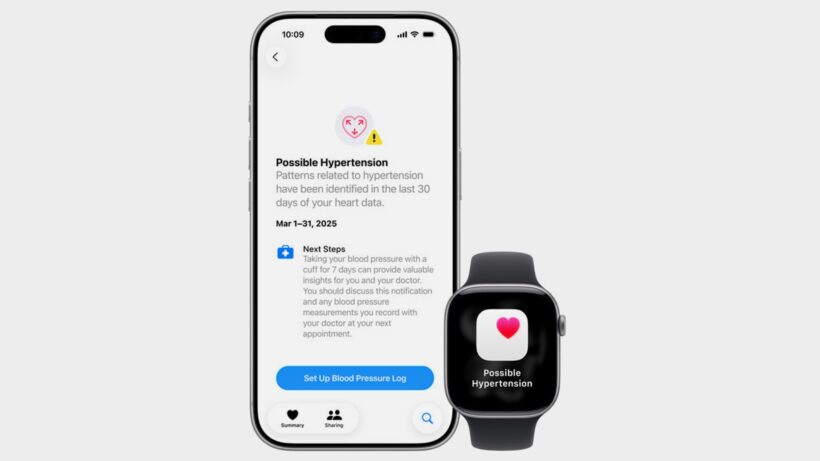
The feature works passively in the background over 30-day periods, using the watch’s optical heart sensor to analyze blood vessel response to heartbeats.
If it detects consistent patterns that may be indicative of chronic high blood pressure, it will send a notification to the user, prompting them to consult with a doctor.
The approvals keep coming
The new clearance is another major regulatory milestone for the Apple Watch, placing the hypertension feature alongside a growing list of validated health tools on the device.
It joins the ECG app, which has been cleared to detect signs of atrial fibrillation (AFib) since 2018, and the more recently introduced features for detecting signs of sleep apnea. The hearing aid feature on AirPods Pro 2, too, received expanded clearance in more regions earlier this year.
This latest approval from the FDA marks another step in the Apple Watch’s development as a serious and credible personal health monitoring device.
We’ll provide a full explainer on the new feature once we’ve had a chance to live with it and understand its nuances. Stay tuned for that.